Composition of the Atmosphere
The present atmosphere of the Earth consists mainly of about 78% nitrogen and 21% oxygen. The biggest minor component is the inert gas argon, making up 0.93%. Components at trace level include carbon dioxide (0.035%) and the inert gases neon, helium, krypton, and xenon (all well under 0.002%).In addition, natural air always contains a certain amount of water vapour. This is often not emphasized (or even mentioned) in giving the composition of air, because it varies strongly with the air temperature, pressure, and humidity, but it is very important.
Saturated air (100% humidity) contains about 0.4% water vapour at freezing point (0 deg C), more than ten times the level of carbon dioxide. At 20 deg C, saturated air contains about 1.7% water vapour, and at 40 deg C, more than 4%.
In Western Australia, air humidity will nearly always reach 100% (making dew form) if the temperature falls to freezing, but will become much lower as the thermometer rises to 40 deg C. Even so, the amount of water vapour in our air will nearly always be over 1%, so that water is the third largest component of our air.
Considerable attention has been paid recently to the level of the fifth largest component, carbon dioxide. This is because of its importance in the 'Greenhouse Effect', which we will look at later. For the moment, we need only note that, at about one-third of one percent, it is really a minor constituent of the atmosphere.
The evidence we shall look at now indicates that this was not always the case. In the past, carbon dioxide was once a major component of the atmosphere. And in the more distant past, it did not figure at all; the Earth's atmosphere has had several complete re-workings in its history, and its present composition bears no resemblance at all to that of the primitive Earth.
Proposition 11A
The composition of the Earth's atmosphere has changed very
markedly at different times in the past, and present and early
compositions are completely different.
Composition of the Early Atmosphere
If the composition of the young Earth's atmosphere was very different, what did it consist of? It is believed that the major components were hydrogen, methane, and ammonia.There are a number of reasons for this belief. It accords well with what is known of the atmospheres of the other planets in our solar system (we will look at this more in NU015). And it does fit in also with the types of sedimentary rocks known to have been formed in the past. For example, the great iron ore deposits of the world are in ancient Pre-Cambrian rocks, and are thought to have been laid down at a time when the Earth had a reducing atmosphere -- an atmosphere with very little oxygen, such that iron would not rust in it like it does in our current oxidizing atmosphere.
Later we will look also at biological evidence. But first we need to look at some of the physical properties of the gases which have been present in the Earth's atmosphere at different times (Table 11).
 Table 11. Gases of the Atmosphere
Table 11. Gases of the Atmosphere
The molecular weight is very important because it has a vital effect on the escape velocity of the gas.
How Gases Work
The physical properties of gases have been studied for some centuries, and are now well-known and easy to understand. Many of these properties, such as change with different temperatures and pressures, depend only on the number of gas molecules present, and not on the type of molecules or mixture of these.Other properties depend directly on the molecular weights of the molecules. These weights are just the sums of the weights of the atoms involved, with the weight of a hydrogen atom, the lightest element, taken as 1. A hydrogen molecule contains two atoms, so its molecular weight is equal to 2.
In a gas, all the molecules are in a state of continuous movement, flying back and forth in every direction. Some are travelling fast, others more slowly -- there is a continuous distribution of speeds, from very fast to stationary. If these molecules are in a closed balloon, they continually beat against the sides of the balloon, exerting a pressure which keeps the balloon extended.
If a gas is heated up, on average its molecules move faster (molecular movement is the same thing as heat, in a gas) and so exert more pressure on the sides. Similarly, if the air in a balloon is squeezed down by outside pressure, there are more gas molecules hitting against a given area of the side, and so the internal pressure rises to match the outside one.
The interesting thing is that a particular volume containing a given number of molecules of a light gas shows exactly the same internal pressure as the same volume with the same number of molecules of a heavier gas. But the weight of the gas depends directly on the molecular weights of the molecules involved. This is why a balloon filled with hydrogen will float; the gas in the balloon is less dense, because it contains the same number of lighter molecules. But to maintain the same internal pressure, the light molecules have to move faster, on average, than the heavy ones.
Flight into Space
The gas molecules making up the Earth's atmosphere are not in an enclosed space, but can mix freely. They beat against the Earth's surface, exerting what is called atmospheric pressure. What about straight up? Why don't they all fly straight off into space?To be able to escape from the planet, gas molecules have to be able to climb out of the Earth's 'gravity well'. Figure 11.1 is a diagrammatic representation of this 'well' -- not a real thing, just a mind model, a means of giving a graphic image of some physical laws.
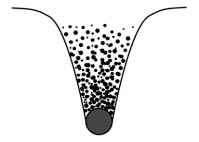 Fig. 11.1. Gas molecules in the Earth's gravity well
Fig. 11.1. Gas molecules in the Earth's gravity well
At the bottom of the well, on the Earth's surface, the gas molecules are thickly clustered and the air is dense. A gas molecule near the bottom which happened to be in flight straight up would most likely collide with another molecule on the way up, but the likelihood of such a collision would be less higher up where the air is thinner and there are fewer molecules around. A rising molecule which did not collide with another would gradually lose speed, as gravity pulled on it, and eventually would stop and fall back to the denser layers.
However, this mind model is called a well because it has a rim. At the rim of the well, the gravity of the Earth is exactly equal to the gravitational forces of the rest of the Universe -- that is what the rim means in this image. If a gas molecule -- or any other object, such as a spacecraft -- is travelling upwards fast enough to be able to reach the rim before gravity saps off all its speed, it can pass the rim and go off into outer space.
Finally we come to the relevance of all this. Molecules of a lighter gas, which on average are travelling faster than those of a heavier gas, are more likely to attain this 'escape velocity' and leave the Earth forever.
Proposition 11B
Light atmospheric gases are much more likely to be lost from
Earth into space than heavy gases
This is not a new Proposition, and not one disputed in any way today, but it is worth making a special point of it now, because of all its implications.
The Snow-Capped Mountains
We need to look now briefly at how air temperature and pressure alter as you go higher. Virtually all the phenomena we experience in the atmosphere, such as winds, clouds and rainfall, and pressure-related weather patterns, exist in the lower layer of the atmosphere, which is called the Troposphere. The troposphere is about 10 km thick (more at the Equator, less at the poles), so even our highest mountains lie within it.Within the troposphere, air temperatures fall as you go higher. There are many somewhat peculiar explanations for this to be found in textbooks, but it is really just a matter of the physics of gases -- the thinner atmosphere higher up needs to be cooler to remain in equilibrium with the denser (and so warmer) layers below. If the effects of all local factors (latitude, season, special topography and so on) are taken out, the rate of fall of temperature with height works out at about 6 deg C/km. This fall is enough to allow snow to remain on the tops of high mountains in the summer, even in tropical areas.
Incidentally, this feature provides another virtually unrecognized reason for temperatures to increase as you go down mines. We looked at this in Chapter 9, where it was suggested that the observed rate of increase in the top 10 km was around 25ºC/ km. We can see now that part of this increase, perhaps around a quarter of it, may be due purely to consequences of the physics of atmospheric gases.
Proposition 11C
Part of the temperature increase observed in going down
mines stems from the same basis of atmosphere gas physics as
that causing a fall in temperature with increasing altitude
Air pressure also decreases as you go higher, a direct consequence of climbing up the gravity well. As well as the more obvious results, a more subtle one comes from the fact that thinner air can 'hold' less water vapour. So there is a double reason for rain or snow to fall on mountains, because the temperatures are lower, and the pressures less.
Another apparently trivial consequence comes from the fact that water boils at a lower temperature under reduced temperatures. Because of this, it is not possible to hard-boil an egg in an open saucepan on the top of Mount Everest -- the water boils below the temperature needed for the biochemical and physical processes involved in hardening the egg. We will return to this trivial point when we consider the fate of the dinosaurs, in NU012.
The Great Reworkings
Apart from the fact that the early Earth's atmosphere probably contained little or no oxygen, but probably held a lot of methane and ammonia, very little is generally agreed concerning the history of our atmosphere. In what follows I will suggest a series of events which lend a much higher degree of detail to what happened.The boundaries between the great geological eras, Pre-Cambrian, Paleozoic, Mesozoic, and Cenozoic, may have been assigned intuitively. But it seems that these boundaries have a physical basis too; they appear to be times of marked change in the atmosphere. In these changes, the role of carbon and its compounds appears to have been a crucial one.
This is not just a physical role. Carbon compounds are the basis of all life on Earth. While physical factors did have an influence, it appears that living organisms were the principal agents in achieving the Great Reworkings of our atmosphere.
The Role of Carbon
The great coal deposits of this planet were laid down in the Carboniferous and Permian periods which ended the Paleozoic. Coal is, of course, mostly carbon, and gave its name to the Carboniferous. Where did the carbon come from? The only credible major source is the atmosphere, presumably by conversion from gaseous hydrocarbons (compounds of carbon and hydrogen, especially methane) or possibly from free carbon dioxide.The massive deposits of high-carbon rocks laid down at the end of the Paleozoic therefore imply a major change in the atmosphere at that time.
The period ending the Mesozoic era, the Cretaceous, was also apparently a time of major atmospheric change. Cretaceous means 'chalky', and chalk and limestone are forms of calcium carbonate, a compound of carbon, oxygen, and calcium. Again, the only credible ultimate source of the carbon is the atmosphere, in this case almost certainly from conversion of carbon dioxide.
The massive deposits of carbonate rock laid down at the end of the Mesozoic era imply a second major change in the atmosphere, involving a massive withdrawal of carbon dioxide from the atmosphere.
The size of the changes involved make them ones of kind, rather than degree. At the present day, estimates are that the ratio of carbon in rock deposits to that in the atmosphere is more than 90,000 to 1 [7], so the amount of carbon left in the atmosphere is very small, only one-hundredth of one percent of the whole. Almost everything that once was in the atmosphere has since been incorporated in the rocks.
Of course, both the coal deposits laid down in the Paleozoic and much of the carbonate deposits of the Mesozoic were formed through the action of living things. Most of the coal came from the giant plants of the Carboniferous swamps, and much of the carbonates from the shells of sea creatures, especially corals and molluscs.
The Primeval Earth's Atmosphere
It has been widely assumed that as part of its formation processes, the Earth inherited a primeval atmosphere consisting mostly of hydrogen, hydrocarbons such as methane, and ammonia. This is reasonable enough.Hydrogen is the most common element in the Universe, and the hydrocarbons are a credible source for the carbon which exists in the biosphere. The ammonia would be the source of the nitrogen in our air. Both carbon and nitrogen are normal products from the nuclear conversion processes which go in stars, and we would expect them to combine with the abundant hydrogen. We will have further evidence in support of this when we look at the atmospheres of other planets, in NU015.
Another common stellar conversion product is oxygen, the most abundant element on Earth. There would also be quite a lot of this, but it would combine immediately with hydrogen to form water. Whether this water would be liquid or vapour would depend on the conditions of heat and pressure on the early Earth.
It is assumed almost as being self-evident that the Earth was originally completely molten. There is, however, little real evidence for this assumption, and it may be that the primeval Earth was never in a particularly hot condition.
Proposition 11D
The primeval Earth was never molten or at a particularly
high temperature
This Proposition appears never to have been examined in any detail, and whether it is true or not, we do not currently have enough evidence to say whether the Earth's initial water was liquid or gaseous. But it must have become liquid fairly early on, because water-deposited sedimentary rocks exist far back into the Precambrian.
We have also seen (NU001) that abundant life on Earth did not appear until the start of the Cambrian, about 600 my ago. However, primitive life existed considerably earlier, as far back as 3500 my ago, through the vast reaches of the Precambrian. What was the nature of this primitive life, and how did it differ from that of today?
Again this is a question on which we have very little evidence, but there is one point of importance. It seems likely that these primitive life-forms lived without free oxygen, and were the forerunners of creatures such as the anaerobic bacteria of today, active in oxygen-free environments such as the bottoms of swamps.
How the Precambrian Ended
We also have very little knowledge of the biochemical processes of these primitive organisms, but it seems possible that they were the motors which drove the conversion of the primitive atmosphere. All the higher forms of life which first appeared in the Cambrian were oxygen-breathers. Those which preceded them were probably not, instead they were lifeforms for which oxygen was a waste product. And in producing this waste product over a period of not thousands or millions of years, but extending to perhaps three billion years, two-thirds of the age of the Earth, they made the oxygen we breathe now.At first all the oxygen they made would be chemically reacted with free hydrogen or the hydrocarbons, but eventually they would have used all this up. And this is the crucial point, the sharp division which marks the boundary between the Precambrian and the Cambrian is probably the time when free oxygen first became common in the Earth's atmosphere. It was this development which permitted the evolution of the oxygen-breathing life which predominates on the Earth today, it was the passing of this threshold which led to the surge in evolution at the start of the Paleozoic.
Proposition 11E
The Precambrian-Cambrian boundary marks the time when
free oxygen first became common in the atmosphere and
permitted
the development of oxygen-breathing life
This was a massive change in the basic nature of the atmosphere. There may also have been changes in the seas as a reaction to this. In particular, the substances dissolved in seawater may have somewhat different.
There are some biochemical differences in lower forms of life which could be traced back to this time, such as the notable percentage of copper in the blood of cuttlefishes, a very ancient line of sea creatures. In more modern creatures this metal is usually replaced by iron. But the most notable change was the one in the shells of shellfish.
The sea creatures of the early Cambrian which possessed shells, skeletons, or other stiffening apparently mostly made this stiffening either out of silica or or calcium phosphate. More modern sea creatures normally use calcium carbonate. In one group of shellfish, the mollusc-like brachiopods, once very common but now rare, a change-over can be traced within the group. Brachiopods which developed at the start of the Cambrian have calcium phosphate shells, but those which had evolved by the end of it have calcium carbonate ones [68].
Perhaps not too much should be made of these differences. However, the seas and the substances dissolved within them have to exist in equilibrium with the atmosphere above, and if this changes in composition, we may expect the seas to be affected also.
Proposition 11F
With the development of free oxygen in the air above the seas,
changes occurred in the composition of substances dissolved in
them
The Paleozoic-Mesozoic Boundary
We have had evidence that the beginning of the Paleozoic was marked by the first significant amount of free oxygen in the Earth's atmosphere and by the appearance of oxygenbreathing life. Now we can look at what happened during the rest of the Paleozoic.Of the 'primeval' atmospheric gases -- hydrogen, methane, and ammonia -- the first would have disappeared completely by the start of the Paleozoic, either completed reacted with the newly formed oxygen to give water, or evaporated off into space. It is likely that the last two would also have undergone transformation during the Paleozoic, the ammonia to form nitrogen and water, and the methane to give carbon dioxide and water. The chemical reactions involved can be easily worked out from Table 11, by adding oxygen to the original gases.
Proposition 11G
Atmospheric ammonia was converted to nitrogen, and methane
to carbon dioxide, during the course of the Paleozoic
There are a few riders to add to this simple statement. The process suggested was unlikely to be sudden, and need not have been complete. There could still have been significant amounts of ammonia and methane left in the atmosphere at the end of the Paleozoic, but the suggestion is that they were no longer major components.
In addition, as already noted, significant amounts of carbon had been taken out of the atmosphere by the end of the Paleozoic to form deposits of coal (and some oil). The only obvious source of this carbon was that in the atmosphere. Whether this carbon was in the form of the original methane or its conversion product, carbon dioxide, is not certain, but it is likely to have been the latter.
Although the question has possibly never been examined in detail, it seems reasonable to assume that the plants which developed on land during the Devonian, Carboniferous, and Permian were at least similar enough to modern ones to be chlorophyll-based. This implies that they gained their energy and substance by photosynthesizing carbohydrates from carbon dioxide, water, and sunlight.
At the same time as the methane was being converted to carbon dioxide, it seems likely that the ammonia was being converted to nitrogen. Apart from the direct changes, there may have been an important indirect effect. Ammonia is alkaline, it dissolves easily in water to form a weak base. Carbon dioxide dissolves in water to form a weak acid, carbonic acid. The implication is that during the course of the atmospheric conversion, the seas changed their state from being weakly alkaline to weakly acid.
Proposition 11H
The Paleozoic-Mesozoic boundary was marked by the
disappearance of methane and ammonia as major
atmospheric components, and the appearance of carbon
dioxide and nitrogen in their place
Proposition 11I
Atmospheric changes at the Paleozoic-Mesozoic boundary
caused a switch in the state of the seas from being weakly
alkaline to weakly acidic
These propositions are supported by observations from the plant world. The lower plants, such as the mosses, often prefer alkaline conditions, and for example can be seen growing on old lime mortar. Moulds grow on shower walls because of the alkaline conditions set up by the regular use of soap. However, higher plants have adapted, and can tolerate a wide range of acid, alkaline, and neutral conditions.
Modern Times: The Mesozoic-Cenozoic Boundary
The Mesozoic was the time of strongest development of life on land. It ended with the death of the dinosaurs and other marked changes in land life. Only with the onset of the Cenozoic do we begin to recognize all the plants and animals as relatives of those we know today.It appears that the atmosphere of the Mesozoic was similar to that of today, with one notable exception. As well as the major components of nitrogen and oxygen, it had a third major component, carbon dioxide.
According to Chambers Encyclopaedia [15], the relative amounts of carbon in different forms on the Earth are as follows (measured in million million tonnes):
| Limestone etc. rocks | 23,100 |
| Dissolved in sea | 22 |
| As coal, oil etc | 6.6 |
| In atmosphere | 0.68 |
| In living plants, timber etc. | 0.009 |
These figures are somewhat different from those in Beckmann [6], who shows rather higher figures for almost all areas except the atmosphere itself (0.58 in 1860, 0.75 now), but the point is very obvious. Hardly any of the Earth's carbon is still left in the atmosphere, it has almost all been withdrawn and deposited in the rocks.
Since we know that a massive part of this withdrawal took place in the Mesozoic, especially in the Cretaceous (the Time of Chalk -- calcium carbonate), it seems almost obvious that the boundary between the Mesozoic and the Cenozoic is the time by which carbon dioxide had ceased to form a significant part of the atmosphere.
Proposition 11J
The Mesozoic-Cenozoic boundary marks the time at which
carbon dioxide levels in the atmosphere had fallen to trace levels
We will return to this point, also, when we consider the Greenhouse Effect in NU017. For the moment we will just comment that this proposition is supported by the fact that modern plants have evolved to be carbon-dioxide hungry; they have produced mechanisms to chase after really quite tiny amounts in the air.
Many commercially-grown vegetables greatly improve their growth in a carbon-dioxide enriched atmosphere, up to five times the normal level (0.15% instead of 0.03%). But at even higher levels, around 0.5%, the carbon dioxide actually seems to become toxic [7]. It would be an interesting exercise, and a test of Proposition 11J, to see if the more ancient plant groups (such as the cycads and ferns) could tolerate a much higher carbon dioxide level.
The Pressure of Earth's Atmosphere
We have seen that there may have been some fundamental upheavals in the composition of the Earth's atmosphere in the past. Now we look at another aspect of the atmosphere: its pressure.The pressure of the air on the Earth's surface is principally governed by two things -- the mass of the atmosphere (how much there is of it) and the forces due to gravity. Once more we will find that the conditions of today are very different to what they may have been in the past.
One of the main factors here is, once more, the carbon dioxide. Working from Beckmann's figures, if all the carbon which he estimates is present in the rocks were in the atmosphere as carbon dioxide instead, this carbon dioxide would weigh some 35 times as much as the whole of the present atmosphere. In other words, if everything else was the same except this carbon was in the air instead of the rocks, we would be living under a pressure of 36 atmospheres.
This might seem incredible, but surprisingly enough, it fits in very well. In NU015 we will see that on Venus, where the atmosphere is mostly carbon dioxide, its pressure at the surface is around 100 atmospheres. And there are several plausible reasons to account for the difference which does exist -- we will look at these, too, later.
The conclusion seems inevitable that atmospheric pressures were very much higher in the Earth's past than they are now.
Proposition 11K
Atmospheric pressures were very much higher on Earth in
the past, because carbon now present in the rocks was formerly
present in the air as atmospheric gases
When we come to consider the effects of Earth expansion, we find that this jump is further compounded. A half-radius Earth would have a quarter of the surface area for the same amount of gas to press on, and so under otherwise equal conditions would have four times the atmospheric pressure, around 144 atmospheres.
Proposition 11L
Atmospheric pressures were also higher in the past because
the same amount of atmosphere was present on a much smaller
Earth
This calculation is obviously only a very first stab at giving a figure to the pressure. If we want to try and put a little more detail into the resulting figures, we need to take into account the period in the past when the carbon was taken out of the air, and its form in that air.
I have already suggested that at the beginning of the Paleozoic, some 600 my ago, the carbon was in the form of methane. Methane has a molecular weight of 16 (Table 11), while carbon dioxide is the heaviest of the gases listed, with a molecular weight of 44. The ratio of weights is 2.75, and since the mass of the atmosphere and hence its pressure on the surface is directly dependent on the weights of its molecules, we would need to divide the above figure of 144 by this ratio. The result, for a methane atmosphere, is around 52 atmospheres.
More Cooking the Books
There are a number of other factors to take into account to try and derive more accurate figures. This is all new ground to break, and here I will just list some of these factors.- Figures for the amount of carbon in the Earth's crust are only estimates, and could be well out. For example, the figures in Beckmann [7] are around twice those in Carbon [15].
- Almost all the carbon in rocks has been deposited since the start of the Paleozoic.
- If methane was being converted to carbon dioxide during the Paleozoic, this would increase the atmospheric mass and pressure.
- Decrease in pressure due to Earth expansion and hence greater surface area would depend on the actual expansion experienced. For example, an Earth of half the present radius may not have been achieved till around 400 my ago, while carbon deposition may have started 600 my ago.
- Substances later converted into atmospheric gases are likely to have been released from the Earth's interior as more surface was exposed, as with the Earth's water (Proposition 10D). These could include carbon dioxide.
- A considerable amount of atmosphere is almost certain to have been lost into space in the past.
The Pea-Soup Scenario
We have now arrived at a set of scenarios for the Earth's earlier atmospheres which differ fundamentally from any proposed elsewhere which I have been able to find. The conventional view is that while the primeval atmosphere contained carbon dioxide and ammonia, these were converted to give an air composition close to that of the present Earth within a few million years after life evolved [21].The scenarios I have presented depict atmospheres of very different composition, in particular of ones containing huge amounts of carbon dioxide compared to today. They are atmospheres of much higher pressures. And they may be ones of very different temperatures.
The higher pressures and temperatures lead to an important consequence which could make the scenarios even more extreme. Air at higher temperatures can hold a lot more water. For example, we have already seen that the amount of water the air can hold increases by a factor of 10 on going from 0ºC to 40º. At a higher extreme, on reaching 100ºC, water evaporates completely under normal pressure, and in theory at least the atmosphere could consist mostly of water vapour at this point.
Similarly, the temperature at which water condenses from steam (same as boiling point) increases markedly with higher pressures. At two atmospheres, it is up to 121 deg C, and at 12 atm it has reached 190 deg C. When the pressure of the air is reduced, as when it rises up to the clouds, it can hold less water and this condenses out as clouds or rain.
At this stage it is very hard to put even tentative figures to the water-vapour content of the Earth's earlier atmospheres, because so much is unknown. But it does seem quite likely that the air contained a great deal more water than it does now.
Proposition 11M
The amount of water vapour held in the Earth's atmosphere
during Paleozoic and Mesozoic times was much greater
than now.
As it would be present in gaseous form, this water would itself increase the atmospheric pressure. Water vapour is one of the lighter atmospheric gases (molecular weight 18), but if even 1% of the Earth's present water was moved into the atmosphere, it would more than triple the atmospheric pressure. Whether such movements in the past have significantly affected sea-levels is too hard to work out at the moment, but it is a possibility worth raising.
We end up with scenarios in which the atmosphere is close to pea soup -- very thick, very moist, very rich in 'Greenhouse Effect' gases. There is another consequence. It may have been perpetually cloudy. The creatures of the Cenozoic, those we start to regard as 'modern', may have been the first on Earth to see the stars.
Proposition 11N
The Earth was completely shrouded in clouds at all times
during the Paleozoic and the Mesozoic
This Proposition is supported by evidence from Venus -- a planet with a high-pressure, carbon-dioxide rich atmosphere, and one perpetually shrouded in clouds. There is also a further implication, relating to nitrogen fixation by thunderstorms.
Thunderbolts of Life
One of the beneficial effects of thunderstorms which is often overlooked is that they fix atmospheric nitrogen into a form which can be used directly by plants. Passage of high-energy thunderbolts through the mixture of nitrogen and oxygen in the air converts some of this to nitrogen oxides, which easily dissolve in water and react to give nitrates, directly usable by plants for food (so-called 'nitrogen-fixing' plants are actually symbiotic associates with micro-organisms which do the actual fixing).Nitrates are a relatively scarce resource for wild plants, and thunderstorms provide quite a significant amount of their needs. In fact they must provide almost all that is not recycled from decaying organisms, either in the same spot or brought in as atmospheric nitrogen gases or dissolved in water. Since it is possible to grow a dense forest containing a lot of nitrogen fixed in its substance from an open field with very little, the nitrogen-transfer activities discussed must be quite significant.
Nitrate fixation through thunderbolts requires a nitrogen-oxygen atmosphere and clouds separated from the planet's surface by a layer of low-conductance air (to allow the build up of an electric-charge potential difference). One or both of these conditions may have been lacking in past eras, so the modern thunderbolt nitrogen-fixing mechanism may have been inoperative.
Proposition 11O
Conditions necessary for atmospheric nitrogen fixing by
thunderbolts were not always present in past eras
Naturally such a situation would have its effects on plant life. In earlier times, there may have been other nitrogen sources -- we have seen that free ammonia may have been much more plentiful in the past. Modern plants are notably 'nitrogen-hungry', especially in our most highly-evolved areas, the tropical rainforests. That is why some of these plants, in the intensely competitive environment, have developed carnivorous habits -- animals are a rich and mobile source of nitrogen.
Flying Creatures of the Past
There is further indirect evidence of a formerly much denser atmosphere, from the Earth's flying creatures. The maximum wingspan of the largest modern flying creature, the albatross, is about 3.5 metres. Creatures tend to evolve to the limits of what is physically possible, and it is unlikely that any modern bird with a wingspan much above 4 metres could survive.However, fossil examples of flying creatures from the Cretaceous, such as the giant pterosaur Quetzlcoatalus alcotaius, are known with wingspans of over 12 m [21], three times this 'theoretical limit'! Similarly, fossil dragonflies have been found with much bigger wingspans than any modern flying insect. Obviously, as the atmosphere thickens, 'flying' moves towards 'swimming', and much bigger wingspans will be feasible at higher atmospheric pressures.
The giant extinct dinosaurs, such as Brontosaurus, were much bigger than any modern land creature, in fact probably bigger than any modern land creature could be (and still move). It has been suggested that creatures like Brontosaurus lived mostly in the water. Could it be that they actually lived on land, but in a much denser and more buoyant atmosphere?
Plants in a Denser Atmosphere
The physical structures of the ancient plants also suggest that they lived in atmospheres much denser than those applying today. The huge trees of Coal Measure times were apparently buoyed up by the dense air, since their cells were large water-filled sacs with comparatively thin walls [68], lacking the strength to stand up under today's conditions.The modern survivors of the most ancient plants, such as the mosses, the ferns, and the cycads, are noted for their affinity for very moist conditions. One of the more primitive of the modern cycads, a Zamia species from the West Indies, actually has sperm which swim in water instead of the immobile pollen of modern plants. The high atmospheric density suggested also fits in well with the mild climatic conditions deduced for earlier times, with no strong winds.
It has often been remarked that the occurrence of the same fossils world-wide in rocks of the Paleozoic and Mesozoic means that conditions must have been much more uniform over the whole world then than they are now. This is true even if it is supposed that, say, fossils now found in the Arctic might have been moved up from warmer areas of origin by domain shifts, because there is no evidence of differing 'cold-weather' and 'warm-weather' fossils for those times, in contrast to modern flora and fauna.
Much more uniform conditions are what would be expected from much denser, moister atmospheres, with higher heat capacities. Temperatures at seaports are much less extreme than those in inland cities, because of the moderating influence of the sea, which is due to its high heat capacity. It would be useful to test whether ancient plants such as the cycads would fare better in much denser atmospheres than modern plants.
All this is positive, but indirect evidence. Wouldn't it be nice if there were direct evidence? And there is.
Truth Frozen in Amber
In an article entitled 'Dinosaur Breath', John Cramer [21] reported on work done by Richard Kerr on the analysis of tiny air bubbles trapped in amber, and the suggested implications of this work. The amber, fossilized resin from extinct species of Pinus, those ancient nut trees, contains tiny air inclusions which Kerr analyzed with a mass spectrometer, an instrument which accurately counts the proportion of different atoms in even very tiny samples.Kerr found that the proportion of oxygen atoms in Cretaceous-amber bubbles 80 my old was much higher than that in modern air, averaging around 30%. On the other hand, the amount of oxygen in bubbles from 40 my-old Cenozoic samples was similar to that in modern air (about 21%).
The conclusion drawn from this was that the Cretaceous air was much richer in oxygen than that of today. Cramer also raises the question of how such large creatures were able to fly during the Cretaceous, and the suggestion given is that the dinosaurs' metabolisms were 'supercharged' by all the extra oxygen, enabling them to overcome the theoretical barriers to flight.
This may be an interesting example of drawing a wrong conclusion from correct data. The point is, that Kerr's technique only counts the number of oxygen atoms, not their chemical state. This excess oxygen could just as well be present as carbon dioxide, which would support the Propositions I have given above. This matter can be tested directly, by re-running analyses to see if carbon from carbon dioxide is present as well.
An even more telling point is mentioned in passing by Cramer. In many cases, the pressure inside the bubbles trapped in the amber was as high as 10 atmospheres. This was attributed to 'the geological forces that converted the pitch to amber'. Is it not more likely that the air trapped in the bubbles was already at a higher pressure? William of Occam would say yes!

(Full list of references at NURefs)
[7]. Roger Beckmann. The greenhouse effect -- not all bad? Ecos/(57) p14-18, 1988.
[15]. Carbon. In: Chambers Encyclopedia/ p89, 1970.
[21]. John G Cramer. Dinosaur breath. Analog Science Fiction/ Science Fact/ July p140-143, 1988.
[68]. F H T Rhodes. A evolucao da vida. Ulisseia, Lisboa, 1960.
NU012: Death Of The Dinosaurs
NU010: The Rolling Oceans
Go to the NUSite Home Page

Version 1.0, in printed edition of "Nuteeriat: Nut Trees, the Expanding Earth, Rottnest Island, and All That...", 1989.
Version 2.0, 2004, PDFs etc on World Wide Web.
Version 3.0, 2014 Sep 10, Reworked from "Nuteeriat" as one article in a suite on the World Wide Web. V. 3.1, Adjusted, 2025 May 31.
V. 4.0, Adjusted for *, 2025 Sep 29.